Vitamin D optimization for GLP-1 success with updated research on deficiency risk, metabolic benefits, and practical dosing strategies for better outcomes.
Table of Contents
Executive Summary
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) such as semaglutide (Ozempic, Wegovy), liraglutide, and others have transformed metabolic disease management — from type 2 diabetes to obesity — by improving glycemic control, reducing appetite, and driving sustained weight loss.
While their metabolic efficacy is well established, growing evidence indicates that nutritional status, particularly vitamin D levels, may meaningfully influence treatment outcomes and overall health status in patients using GLP-1 therapies.
This guide synthesizes the most current research and clinical best practices as of 2026 and provides actionable recommendations for clinicians, allied health professionals, and informed patients.

Why “Normal” Isn’t Enough in 2026
Most standard lab tests list 30 ng/mL as the baseline for Vitamin D.
However, for those undergoing rapid metabolic shifts, Vitamin D Optimization requires a much higher threshold.
In the context of GLP-1 therapy, we aim for the “Vitality Range” of 50–80 ng/mL.
Why? Because Vitamin D receptors (VDR) are located directly on the hair follicle.
When levels drop, the follicle enters a “dormant” state, contributing directly to the thinning we discuss in our comprehensive guide to GLP-1 hair loss.
1. Background: GLP-1 Receptor Agonists in Metabolic Therapy
GLP-1 RAs mimic the endogenous incretin hormone GLP-1, enhancing insulin secretion, suppressing glucagon, slowing gastric emptying, and promoting satiety.
These mechanisms drive significant improvements in glycemic control for people with type 2 diabetes and clinically meaningful weight loss in individuals with obesity or overweight.
The benefits have been sustained in long-term clinical use, with semaglutide trials showing maintenance of improved body weight and glycemic outcomes for up to three years.
However, appetite suppression and reduced food intake — central features of effective GLP-1 therapy — can inadvertently impair intake of micronutrients, including vitamin D.
Several observational and retrospective studies now highlight the incidence of nutrient deficiencies among GLP-1 RA users.
2. Incidence and Clinical Importance of Vitamin D Deficiency on GLP-1 Therapy
A retrospective observational cohort study analyzing over 460,000 adults treated with GLP-1 RAs documented that nutritional deficiencies are common during the first year of therapy, with vitamin D deficiency emerging as the most frequent.
Within 6 months, approximately 7.5% had confirmed vitamin D deficiency; by 12 months, the incidence rose to nearly 13.6%.
Muscle loss and anemia also accompanied these deficiencies.
Cross-sectional nutritional assessments of patients on GLP-1 RAs similarly confirm intake of vitamin D significantly below reference recommendations.
In one cohort, average dietary vitamin D intake was approximately 4 mcg per day — far below recommended intakes — alongside low intake of other micronutrients.
Clinical significance:
- Vitamin D is essential for bone mineralization, immune regulation, and muscle function.
- Deficiency is linked with increased risk of bone loss, falls, and fractures; in metabolic syndromes, low vitamin D correlates with insulin resistance and impaired glucose metabolism.
- Observational research has identified a positive correlation between serum vitamin D and endogenous GLP-1 levels in elderly patients, suggesting potential interplay in metabolic regulation.
Clinical and Observational Evidence Linking Vitamin D Status with GLP-1 Therapy or GLP-1 Biology
Given these intersections — GLP-1 therapy, nutritional changes, and metabolic health — optimizing vitamin D is now recognized as an important component of comprehensive clinical care.
| Study / Reference | Study Design & Population | Exposure / Intervention | Key Vitamin D–Related Outcomes | Relevance to GLP-1 Therapy |
|---|---|---|---|---|
| Butsch et al., 2025 (N=461,382) | Retrospective cohort of adults initiating GLP-1 receptor agonists (GLP-1RAs) | Routine clinical use of GLP-1RAs (various agents) | Vitamin D deficiency was the most common micronutrient deficiency diagnosed: 7.5% at 6 months, 13.6% at 12 months post-initiation. | Demonstrates high prevalence of vitamin D deficiency in real-world GLP-1RA users, highlighting the need for nutritional monitoring. |
| Cross-sectional dietary study (N=69) | Cross-sectional analysis of adults on GLP-1RAs | Self-reported nutrient intake + three-day food records | Mean intake of vitamin D was 4 mcg/day, substantially below Dietary Reference Intakes (indicating insufficiency). | Supplements the above findings with dietary intake data, showing inadequate vitamin D consumption among GLP-1 users. |
| In elderly fracture patients (N≈87) | Case–control biochemical study | Serum measurements of vitamin D and GLP-1 | Positive correlation between 25(OH)D levels and GLP-1 levels; both were lower in fracture patients vs controls. | Suggests a biologic association between vitamin D status and GLP-1 physiology (not specific to GLP-1 therapy but relevant to metabolic/endocrine interplay). |
| GLP-1 use in multiple sclerosis cohort | Retrospective observational (MS patients) | GLP-1 initiation vs baseline | Post-GLP-1 initiation vitamin D levels increased by ~8.1 ng/mL alongside weight loss. | Indicates that weight loss itself can improve circulating vitamin D in certain populations, possibly due to adipose release and metabolic changes with GLP-1 therapy. |
3. Physiology of Vitamin D: Why Status Matters
Vitamin D exists in two primary forms:
- 25-hydroxyvitamin D (25[OH]D): The major circulating form, used to assess status.
- 1,25-dihydroxyvitamin D: The active hormonal form.
Vitamin D influences multiple physiological systems relevant to GLP-1 therapy outcomes:
A. Calcium Homeostasis and Bone Health
Adequate vitamin D promotes calcium absorption and bone remodeling — important for patients experiencing weight loss, which can accelerate bone resorption if nutrient status is poor.
B. Immune Health and Inflammation
Vitamin D modulates immune responses and may blunt chronic inflammation — a feature of obesity and metabolic syndrome, potentially enhancing metabolic flexibility.
C. Glucose Metabolism
Vitamin D receptors (VDRs) are expressed in pancreatic β-cells and muscle tissue.
Independent meta-analyses suggest vitamin D supplementation can modestly improve fasting glucose, HbA1c, and insulin sensitivity in at-risk populations (e.g., prediabetes), especially at higher doses and in vitamin D–deficient individuals.
D. Influence of Adiposity on Vitamin D
Body fat can sequester vitamin D, decreasing its bioavailability.
Weight loss induced by GLP-1 therapy may increase serum vitamin D concentrations, especially in women, likely reflecting release from adipose stores.
4. Optimal Vitamin D Targets for Patients on GLP-1 Therapy
To optimize Vitamin D levels as of 2026, medical research emphasizes maximizing bioavailability and maintaining serum concentrations above 30 ng/mL.
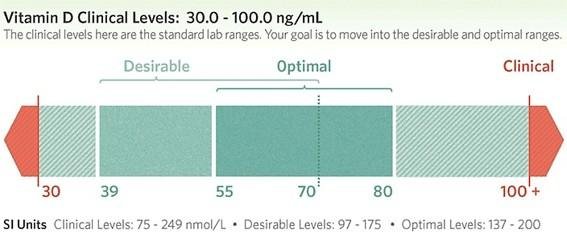
Clinical guidelines recommend maintaining serum 25(OH)D levels within an optimal range to support bone and metabolic health. Although specific targets may vary by guideline body, a widely accepted framework includes:
- Deficiency: < 20 ng/mL
- Insufficiency: 20–30 ng/mL
- Sufficiency (optimal): 30–50 ng/mL
(For detailed guidance on vitamin D status and supplementation, refer to the NIH Office of Dietary Supplements Fact Sheet on Vitamin D.
Maintaining optimal levels is especially important for patients with obesity, limited sun exposure, dark skin pigmentation, or malabsorption conditions — all factors that impair vitamin D synthesis or bioavailability.
5. Practical Strategies for Vitamin D Optimization

A. Baseline and Ongoing Monitoring
- Baseline 25(OH)D assessment prior to or at GLP-1 therapy initiation.
- Follow-up testing every 3–6 months in patients with deficiency or high risk of deficiency.
- Coordinate assessment with other micronutrients (calcium, magnesium, B vitamins) that interact with vitamin D metabolism.
B. Dietary Sources
Encourage consumption of vitamin D–rich foods:
- Fatty fish (salmon, mackerel, sardines).
- Fortified dairy and plant milks.
- Egg yolks and fortified cereals (note: intake may be limited in GLP-1 patients due to appetite suppression).
However, many individuals do not achieve adequate intake through diet alone.
C. Supplementation
Supplementation should be individualized based on baseline levels, risk factors, and clinical context. Typical dosing strategies include:
- Deficiency correction: Higher initial doses (e.g., 2,000–5,000 IU/day) under medical supervision until levels normalize.
- Maintenance dosing: 800–2,000 IU/day commonly recommended for adults, adjusted based on laboratory follow-up and clinical factors.
Higher dosing may be considered in consultation with a clinician, especially in patients with significant deficiency.
D. Sun Exposure
Safe sun exposure (e.g., 10–30 minutes, several times per week) can contribute to vitamin D synthesis; however, latitude, season, skin pigmentation, and sunscreen use all modify effective synthesis.
E. Addressing Absorption Barriers
Patients with gastrointestinal malabsorptive conditions may require specialized formulations (e.g., oil-based supplements) or higher dosages for effective repletion.
6. The Three Pillars of Vitamin D Optimization
1. The VDR Sensitivity Factor
Recent 2026 research shows that GLP-1 medications can temporarily alter how your receptors respond to hormones. Vitamin D Optimization ensures that even if receptor sensitivity dips, there is enough circulating calcitriol to keep your metabolic engines running.
2. Magnesium: The Silent Partner
You cannot achieve Vitamin D Optimization without Magnesium. Magnesium is the “workhorse” that converts inactive Vitamin D into its usable form. If you take high-dose D3 without Magnesium, you may actually deplete your mineral stores, leading to the muscle cramps often listed in our guide to Semaglutide side effects.
3. The Fat-Soluble Synergy (K2)
To ensure the calcium mobilized by Vitamin D goes to your bones and hair—and not your arteries—you must pair your supplement with Vitamin K2 (MK-7). This “triad” of D3, K2, and Magnesium is the foundation of the Micronutrient Shield.
7. Vitamin D, Muscle Health, and Body Composition
Rapid weight loss can enhance vitamin D status as fat mass decreases, but lean muscle preservation remains a concern.
Muscle weakness and loss are documented complications among GLP-1 RA users with inadequate nutrient intake, potentially compounded by low vitamin D.
Maintaining adequate levels of Vitamin D supports muscle function and may mitigate sarcopenia associated with aggressive weight loss or aging.
Dosage:
- Maintenance: A daily intake of 1,000 to 2,000 IU is often recommended for most adults without a deficiency.
- Deficiency Correction: Higher doses, such as 4,000 IU daily, may be necessary under medical supervision to correct an existing deficiency.
8. Integration With GLP-1 RA Clinical Care Pathways
Vitamin D optimization should be integrated into the clinical workflow of GLP-1 therapy:
Pre-Therapy Assessment
- Comprehensive metabolic panel.
- Nutritional assessment including vitamin D.
During Therapy
- Regular monitoring and dietary counseling by a registered dietitian to preempt deficiencies.
- Adjust supplementation based on serum levels and clinical response.
Multidisciplinary Collaboration
- Work with endocrinologists, primary care providers, dietitians, and exercise specialists to align nutritional, metabolic, and therapeutic goals.
9. Evidence Gaps and Future Research Directions
While mechanistic rationale and observational data suggest a beneficial role for maintaining adequate vitamin D in patients using GLP-1 RAs, robust randomized controlled trials specifically examining the effect of vitamin D optimization on GLP-1 therapy outcomes (e.g., weight loss durability, glycemic control, body composition) remain limited.
Ongoing and planned clinical investigations — including those assessing baseline vitamin D status as a moderator of semaglutide effectiveness — will clarify causal effects.
Clinicians should remain cognizant of emerging evidence and adapt practices accordingly while adhering to established nutrient guidelines.
FAQ – Vitamin D Optimization (Frequently Asked Questions)
Conclusion – Vitamin D Optimization
Optimizing vitamin D status is critical for individuals on GLP-1 medications due to a high prevalence of pre-existing deficiency in obese patients and a heightened risk of nutritional gaps during treatment.
Vitamin D optimization involves achieving blood levels (25(OH)D) generally between 30-60 ng/mL, often targeting 40-50 ng/mL for broader health.
Adequate vitamin D supports metabolic health, bone density, and muscle function, which are key concerns during rapid weight loss.
Given the high prevalence of deficiency among patients undergoing appetite-modulating treatment, proactive assessment, monitoring, and individualized supplementation, incorporated into comprehensive care plans, can help mitigate risks associated with deficiency and support long-term therapeutic success.
Authoritative Sources
- NIH Office of Dietary Supplements — Vitamin D Fact Sheet for Consumers
- American Diabetes Association Standards of Care (2026) — Nutrition and Dietary Supplements in Diabetes Management: